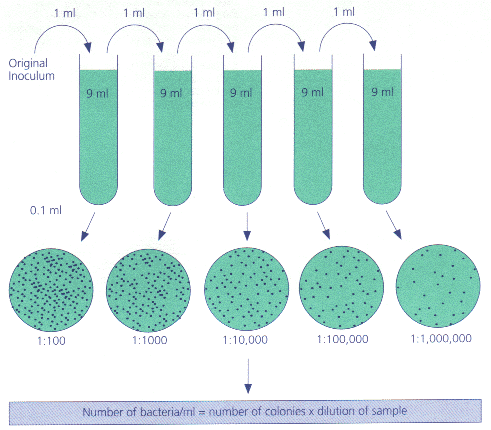
Serial Dilution Cells

A serial dilution is the stepwise dilution of a substance in solution. Usually the dilution factor at each step is constant, resulting in a geometric progression of.

Serial dilution
May 19, 2008 How to perform a serial dilution of chemicals in the lab.
The MicrobeLibrary includes peer-reviewed visual resources and laboratory protocols for undergraduate microbiology and science education supported by the American.
Sep 06, 2013 Understand how to calculate a serial dilution This feature is not available right now. Please try again later.
The story on the previous pages has many parallels with life in a microbiology lab. Frequently, you will find it necessary to add water or some other medium to a stock or soup, get it. with a known concentration to make a more dilute solution.
Why would you want to do this. Let s say you need to get 1/10,000th of a mL in order to count the bacteria in it. That would be pretty difficult with a pipette. But instead, you could
Take that 1 mL and put it in 99 mLs of saline solution. You still have the same number of bacteria, but now they re spread out.
Now you can take 1 mL of THAT solution, add it to a new container, top off with 99mLs of water, and you ll have only 1/100th of the original bacteria in the mL.
Pull out 1 mL of this latest mixture, and you ll have 1/100th of 1/100th, which is multiplying the fractions together 1/10,000th.
When you do serial dilutions, you multiply together all of the dilution factors. Make sure you are clear on what constitutes a dilution factor. When I add a small amount of the concentrated stuff to an empty container, top it off with saline/water/whatever, then remove some small amount, this constitutes a dilution. So above, even though its broken down into 3 steps, there are really only 2 full dilution steps.
When in doubt, try to think it through logically. Often it helps to think through the whole process using some concrete number. For example: I started with 300,000 cells in a mL, put those into 99 mLs of saline, and took out a mL, so there must have been 3,000 cells in that mL. Then I put those 3,000 cells into 99 mLs of saline, and took out one mL again, so there must have been 30 cells in that mL. Overall my dilution must have been to 30 from 300,000, which is the same as to 1 from 10,000 ,000. Of course, the idea that you started with 300,000 cells is pure fiction, but it can help you make sure that you ve done the dilutions correctly.
Put one mL of a stock into 99 mLs of water. Take 1 mL of that and put it in 999 mLs of water. What is the total dilution.
To make this problem interactive, turn on javascript.
I need a hint :Assume that you start with 1,000,000 cells in one mL of solution
another hint :After first dilution, you are left with 10,000 cels in one mL of solution
another hint :After second dilution, you are left with 10 cells in one mL of solution
another hint :Multiply two dilution factors to get the total dilution
I think I have the answer: 0,000
Start with a 00 dilution someone else has made. Take 1 mL and put it in 99 mLs of water. What is the total dilution.
I need a hint :Assume that you start with 10,000 cells in one mL of solution
another hint :After first dilution, you are left with 100 cells in one mL of solution
another hint :First dilution factor is 0
another hint :Multiply the dilution factor you obtained with the initial dilution factor to get the total dilution
Put one mL of a stock into 49 mLs of water. Take 1 mL of that and put it in 49 mLs of water. What is the total dilution.
I need a hint :Assume that you are starting with 20,000 cells in one mL of solution
another hint :After first dilution you are left with 400 cells in one mL of solution 20,000/50
another hint :After second dilution you are left with 8 cells in one mL of solution 400/50
another hint :Multiply two dilution factors 1/50 1/50 to get the total dilution
Put one mL of a stock into 99 mLs of water. Repeat 3 more times. What is the total dilution.
I need a hint :Assume you are starting with 1,000,000,000 cells in one mL of solution
another hint :After first dilution you are left with 10,000,000 cells in one mL of solution dilution factor 0
another hint :Dilution factor is the same each time
another hint :Multiply all four dilution factors to get total dilution
I think I have the answer: 0,000,000
Start with a ,000 dilution someone else has made. In order to get a 0,000 dilution, you need to put 1 mL into __ mLs of water.
I need a hint :Determine the dilution factor to get 0,000 dilution from ,000 dilution
another hint :Dilution factor is
another hint :You need to have 10 mL of solution to get 0,000 of dilution
Starting with a dilution made by a TA, you add 1 mL to 49 mLs of water to get a 0,000 dilution. What was the TA s dilution factor.
I need a hint :Your dilution factor is
another hint :Divide final dilution with your dilution factor to get initial dilution
I think I have the answer: ,000
If we put dilution and scaling up together, we can estimate the number of bacteria in the original sample. For example:
If a ,000 dilution results in a plate with 176 CFUs, then the original number of bacteria in the sample.
I need a hint : Remember you have basically counted 1/10,000th of the original bacteria.
another hint : Multiply by the reciprocal of the dilution factor
one more hint : Don t forget that we re only reporting 2 significant digits.
I think I have the answer: approximately 1,800,000.
Pretty easy, right. The only tricky part is that you don t know in advance how far to dilute your original sample. Let s see what happens if you always count a ,000 diluted sample. If your original solution contained
50,000,000 bacteria: the ,000 sample will contain about 5,000 CFUs. You would be counting all day and the CFUs overlap and it s a mess.
500,000 bacteria: the diluted sample will contain about 50 CFUs, which is both easy to count and valid for scaling up.
50,000 bacteria: the diluted sample will contain ON AVERAGE 5 CFUs. But remember that diluting and sampling is bascially a random process, and imagine the havoc caused by one extra CFU ending up in your pipette -- suddenly you estimate the population as 60,000 rather than 50,000, and you re off by 20.
5000 bacteria: the diluted sample will contain ON AVERAGE 0.5 CFUs -- meaning that any given sample will probably contain either 0 or 1 CFU. This will be easy to count, but its not valid for scaling up.
So here s the general rule: in order to be valid, the plate that you scale up should contain between 25 and 250 CFUs. Any more than 250 CFUs can cause overlap, and you ll underestimate the population size. Any fewer than 25 makes your estimate vulnerable to over- OR under-estimates based on random chance alone.
So the real trick here is picking the correct dilution. For the 50 million bacteria case, 1:1,000,000 would have been a good dilution facter. For the 5 thousand bacteria case, 0 would have done the trick. And since you can t know ahead of time what that is, instead you ll need a series of dilutions.
A series of dilutions ensures that one of these dilutions will be in the countable range 25 to 250 CFUs.
So let s put this all together. We would like to count the bacteria in a particular sample, and we think that there should be in the neighborhood of 10 million cells per mL.
Ideally, therefore, we would like to get 100 bacteria on the plate that we count. That would be a dilution factor of 1,000,000, or 0,000. And to give ourselves a little wiggle room, we should start at least 1 dilution before that, so ,000. Then we ll do three more dilutions to get our series.
In the table below, click on the button corresponding to the plate that should be used to estimate the original concentration of bacteria:
Pretty straightforward, right. Below, you can practice a couple more times. I also included a few unusual situations things do go wrong, of course, and you should be able to recognize that. If you think the dilution series is not valid, click on re-do the dilution.
Before you head off, I just want to say a few words about resolution.
Even if you have perfect technique in mixing and pipetting, you cannot determine the exact number of bacteria present in your sample. This is an unavoidable consequence of diluting and scaling up.
Let s imagine that we have a sample with exactly 183,000 cells per mL. And let s say that our technique is flawless. If we count a ,000 diluted plate, we should find 183 CFUs remember, we have magically flawless technique. But the exact same thing would happen if our sample started with 183,001 cells. Or 183,100 cells. Or 183,400 cells. Or 182,800 cells. In fact, even with our absolutely flawless technique, the best we can say is that there are between 182,500 and 183,500 cells. So our absolutely unavoidable error is plus or minus 500/182,000 -- about 0.27. That s pretty good.
Let s look at some other ways we could count the same 183,000 cell sample:
So you can see that the 25 to 250 guideline is really a compromise between the ability to distinguish non-overlapping CFUs, and the resolution afforded by the scaling up component.
In the applet below, you can practice the whole process of serial dilution from beginning to end. If you don t get a good count the first time, try it again.
Hint: this applet previews the module on bacterial growth rates a bit. Specifically, you need to know that the doubling time is the amount of time it takes a population to double. So if you start with a population of 100 and it doubles every 10 minutes, after a half hour it has doubled 3 times to about 800.
Scaling up is technique used to estimate the size of huge population of bacteria based on the calculations from smaller sample.
Serial dilution is a step by step dilution of concentrated solution into diluted solution, where each dilution gets you closer to your goal.
You can multiply together all of the dilution factors to get total dilution factor.
It is very important to dilute sample up to a certain extent, so that the number of cells/bacteria are in countable range. If after a dilution, the number of cells/bacteria is between 25 and 250, they are both easy to count and valid for scaling up.
It is also important to know that even if you have perfect technique in mixing and pipetting, you can only estimate the number of bacteria present in your sample. It is impossible to know the exact number of bacteria.
If you want a printer-friendly version of this module, you can find it here in a Microsoft Word document. This printer-friendly version should be used only to review, as it does not contain any of the interactive material, and only a skeletal version of problems solved in the module.
This module is currently under revision
In this module, we re going to consider some practical implications of counting bacteria. Primarily, we re going to deal with 2 questions:
how do I count something that runs into the millions or billions.
We ll talk about a specific method serial dilution, plating, counting, and scaling up, but I want to make it clear at the outset that the problem of counting huge numbers of things is not at all unique to counting bacteria. Many of the same issues scaling up, error propagation apply to other things that people want to count, like insects, birds, contaminants, pollutants, human population
So, we re going to start by talking about scaling up.
Richard Feynman, one of the most celebrated physicists in modern times, was famous for beginning his first-year honors physics class at Caltech by asking the students to estimate how many barbers work in Chicago. Presumably he was not interested in getting a haircut. Instead, he was in part asking his students to scale up an estimate based on small sample.
So, for example, you might estimate that in one square urban mile there is on average one barber shop just barbers, not hairdressers with 2 barbers. And the area of Chicago is about 2100 square miles according to Wikipedia. So,
if there are 2 barbers per square mile, and
then how many barbers are there in Chicago.
The answer of course is 2100 2 approximately 4200. And in fact you would be approximately right. According to one website, there are 4140 barbers in Chicago in early 2006 -- and according to that same website, this is a question sometimes used in job interviews to determine how well candidates think on their feet. Talk about far-reaching applications of mathematical concepts.
How many plastic shopping bags are used per year in the U.S..
Clearly this is not something you want to go out and count. But without doing a few calculations, its impossible to make even an informed guess. Is it billions. trillions. more.
Instead, try making an informed guess, based on your own use of plastic bags, and a US population of 300 million people
I need a hint : How many bags do you use per day. For example, if you shop for groceries once a week and go to some other stores daily, you might use about 4 bags per day.
another hint : You need to multiply your own use by 365 300 million.
I think I have the answer: your daily use one hundred billion
In fact, according to reusablebags.com, the number is about 4.6 10 11, or 460 billion.
Notice that I am being a little cavalier with the numbers here. I say I use about 4 bags a day, and multiply by about one hundred million. Not only is it OK to be a little vague, its absolutely necessary, otherwise you re claiming more knowledge than you actually have. Saying:
Americans use about 4.6 hundred billion bags/yr
is a reasonable statement. But saying
Americans use 304,339,593 365 4 444,335,805,780 bags/yr
is ridiculous, and it makes you sound like a pompous know-it-all to boot. For one thing, the U.S. population has changed since I wrote the above equation. For another thing, 4 bags a day was only a rough guess. And technically years have 365.25 days.
So I really don t know how many bags were used, but if I did enough research, I hope to be able to say between 4 and 5 hundred billion, or even in the hundred-billion range. In other words, not trillions, not millions, but hundreds of billions.
The next problem is easy, but don t skip it:
How many m m s are there in a one-pound bag, if a bag weighing 1/11 of a pound has 35 m m s.
I need a hint : 11 of the little bags would go into one big bag
I think I have the answer: about 35 11 about 400 m ms.
Yes, I know 35 11 is 385, but do you really want to claim that a pound of m m s contain exactly 385 pieces. Based on me eating -- I mean, counting -- a single fun-size pack.
Also, notice in this problem I didn t tell you how much the small bag weighed, only that it contained 1/11 of the amount of a large bag. Still, you probably found it easy to determine that you needed to multiply by 11. This is called multiplying by the reciprocal.
Reciprocal, in this case, means what you get when you flip a fraction upside down. It s the over part of one over eleven.
And yes, this is a biology class :
Some students are measuring bacterial contamination. They take a very small sample of the contaminated food -- about one-ten-thousandth 1/10,000 of the container -- and count 49 bacterial cells. How many bacteria were in the original container of food.
I need a hint : The students took a 1/10,000 sample, so what do they need to multiply by.
another hint : Multiply the number found by the reciprocal of 1/10,000
I think I have the answer: about 490,000.
Again, when we say about 490,000, we really mean more than 485,000 but less than 495,000. There are statistical ways to determine exactly what range you think the anwer lies in, but we ll save that for a different module.
If you found this last problem easy, that s great. Multiplying by the reciprocal of your sample size is the heart of counting bacteria by the serial diluation method. The rest is just technique.
Speaking of technique just how did those students get a sample of 1 ten-thousandth of the bacteria in their food. I know kids have a hard time just dividing a cookie into 3 equal pieces. Well, that s where the technique comes in. Basically, it s awfully hard to divide something into teeny-tiny pieces unless you first blow that something up and make it huge. That s what the story on the next page is about
Once Upon a Time, there was an unbearably snobby queen-mother with a son of marriageable age. The son was smart, charming, and funny, but not one of the princesses that he brought home was good enough for his mother. One day, while daydreaming in his calculus lecture, the prince met his true love. He was giddy with emotion, but realized that convincing his mother would take some work.
When the prince and his intended arrived at the palace for spring break, he found his mother uncharacteristically in the kitchen. I ve made your favorite, cooed his mother unconvincingly, split pea soup. My very own recipe. 9 million very small peas per liter. I m sure your little friend will love it. But if not, well, not everyone is made for royalty.
The prince looked at the mushy glutinous mass in his mother s cauldron with dismay. He knew that his relationship was doomed if he didn t find a way out. He racked his brain for solutions, but his courseload of calculus, microbiology, organic chemistry, and analysis of Swiss yodeling had left little room for culinary improvement. In other words, he could barely boil an egg. Frantically he considered derivatives, limit theorems, integrals and natural logarithms, but they all seemed a bit, well, complicated, given that dinner was to be served in six minutes and his mother was out adding a fifth spoon to every place setting.
The prince s sister dilutes the soup
Just then his younger sister entered the kitchen and saw his miserable face. When he explained the predicament, she just smiled and said all you need is plain old multiplication. In vain the prince tried to explain that he needed to dilute the soup, not multiply it. Exactly. said his sister. Anyone can eat a soup that has 90 peas per liter. All we have to do is figure out how to get it diluted to that level. So
That s a dilution factor of 100 to 1. That way, each liter of soup will have only 90,000 peas instead of 9 million.
Yeah, but we ll also have 100 liters of soup. There s not enough tupperware here for all that food.
OK, that s a good point. I didn t think of that Well, we don t need to mix up all 100 liters. All Mumsy expects is a single pot. And all that matters is that we get the ratio right hmm let s say we started with a hundredth of a pot of the old soup and added 99 hundredths of a pot of water.
What do we do with the rest of the soup.
Um, keep it for your next girlfriend.
Luckily, the kingdom measured in metric only. One soup pot held 1 liter of soup, so the sister removed 10 mLs and put it into a clean pot. She then filled the pot to the brim with water. Voila, soup with only 90,000 peas per liter.
The prince gave it a try and promptly spit it back out again. Blaaah, still too much. Its nasty he said, just as his second sister came in. A quick conference between sisters brought the second sister up to speed. Let s do the same thing again. said the second sister enthusiastically.
Great, it didn t work the first time, so now we re going to do it again.
Yes, we already got it partway diluted, now we ll just dilute it some more.
So Sister 2 took 10 mL of the diluted soup, put it in a third pot, and filled that pot to the brim with water.
The prince gave the double-diluted soup a try. Still too strong, he muttered. Cue Sister 3, another explanation of soup, mothers, and true love, and you should be able to see it coming a third dilution. But this time, going from 900 to 90 peas per liter required only a ten to 1 dilution. Hence the third sister put 100 mLs of soup one tenth of the total into a new pot, and filled the pot full to the liter mark:
The princess drinks the thrice-diluted soup.
The prince tried the triple-diluted soup. Wow. Fantastic. It tastes just like water.
At dinner the prince s girlfriend slurped down the watery soup like a trooper.
Sadly, the prince s mother was not blind or totally stupid. She quickly caught on to the soup-dilution scheme, found the leftover stock, dumped it over the prince s head, and sent the girlfriend packing. The prince passed calculus, graduated with slight honors, and lived out his life in bitter isolation. Moral of the story: don t mess with mumsy.
OK, there might be a few other morals:
Serial dilution means you do a series of dilutions, where each dilution gets you closer to your goal.
Each dilution can be seen as a conversion of a concentrated stock into a diluted stock. This is pretty obvious when you re dealing with factors of 10 or 100, but could be trickier if you had to use less comfortable numbers.
It doesn t really matter how much stock you start with, the important thing is to take out the right fraction of it a tenth, a hundredth and then add enough water to get back to the initial volume.
Serial Dilution: How it works. The story on the previous pages has many parallels with life in a microbiology lab. Frequently, you will find it necessary to add.
It is a common practice to determine microbial counts for both liquid and solid specimens---suspensions of E. coli in nutrient broth all the way to soil samples and hamburger meat. Most specimens have high enough numbers of microorganisms that the specimen has to be serially diluted to quantitate effectively.The following is a step-by-step procedure to working dilution problems, and includes some practice problems at the end.The purpose can be determination of bacterial, fungal, or viral counts. This protocol is specific for bacterial counts colony-forming units, CFUs, but can be modified for fungi CFUs and viruses plaque-forming units, PFUs for viral counts. HistoryRobert Koch is credited with identifying a method for bacterial enumeration, used first for the study of water quality. His article, About Detection Methods for Microorganisms in Water. was published in 1883.The standard plate count is a reliable method for enumerating bacteria and fungi. A set of serial dilutions is made, a sample of each is placed into a liquefied agar medium, and the medium poured into a petri dish. The agar solidifies, with the bacterial cells locked inside of the agar. Colonies grow within the agar, as well as on top of the agar and below the agar between the agar and the lower dish. The procedure described above produces a set of pour plates from many dilutions, but spread plates sample spread on top of solidified agar can be used also. The agar plate allows accurate counting of the microorganisms, resulting from the equal distribution across the agar plate. This cannot be done with a fluid solution since 1 one cannot identify purity of the specimen, and 2 there is no way to enumerate the cells in a liquid.
PrinciplesTHE STANDARD FORMULA colony count CFUs on an agar plate
total dilution of tube used to make plate for colony count X volume plated To work the problem, you need 3 values---a colony count from the pour or spread plates, a dilution factor for the dilution tube from which the countable agar plate comes, and the volume of the dilution that was plated on the agar plate.PROTOCOLSTEP 1: Determine the appropriate plate for counting:Look at all plates and find the one with 30-300 colonies see COMMENTS TIPS section at end for explanation.
Use the total dilution for the tube from where the plate count was obtained.
If duplicate plates with same amount plated have been made from one dilution, average the counts together. STEP 2: Determine the total dilution for the dilution tubes:Dilution factor amount of specimen transferred divided by the total volume after transfer amount of specimen transferred amount of diluent already in tube. Determine the dilution factor for each tube in the dilution series.
Multiply the individual dilution factor for the tube and all previous tubes. To calculate this dilution series:
Determine the dilution factor of each tube in the set.dilution factor for a tube _____amount of sample________
volume of specimen transferred volume of diluent in tubeBut after the first tube, each tube is a dilution of the previous dilution tube.SO. .total dilution factor previous dilution factor of tube X dilution of next tubeFOR THE ABOVE DILUTION SERIES:0.5 ml added to 4.5ml 0.5/5.0 5/50 1/10 for 1st tube1ml added to 9ml 1/10 2nd tube X previous dilution of 1/10 1st tube total dilution of 1/100 for 2nd tube.STEP 3: Determine the amount plated the amount of dilution used to make the particular pour plate or spread plate. There is nothing to calculate here: the value will be stated in the procedure, or it will be given in the problem.STEP 4: Solve the problem
1. The countable plate is the one with 51 colonies.2. The total dilution of the 2nd tube from which that pour plate was made 1/102 3. The amount used to make that pour plate 0.1ml convert to 1/10 - it is easier to multiply fractions and decimals together. 51 colonies 51 X 103 5.1 X 104 scientific notation OR 51,000 CFUs/ml
1/102 X 1/1045 colonies 45 X 104 4.5 X 105 scientific notation OR 450,000/ml
1/103 X 1/10 SAFETYTubes and agar plates should be discarded properly in a biohazard container for proper sterilization. The pipettes will also be sterilized washed first if using reusable glass pipettes.Do not pipette by mouth.Use sterile technique in the transfer of microorganisms from tube to tube, as well as in the production of the pour plates.
The ASM advocates that students must successfully demonstrate the ability to explain and practice safe laboratory techniques. For more information, read the laboratory safety section of the ASM Curriculum Recommendations: Introductory Course in Microbiology and the Guidelines for Biosafety in Teaching Laboratories.
COMMENTS AND TIPSGreater than 300 colonies on the agar plate and less than 30 leads to a high degree of error. Air contaminants can contribute significantly to a really low count. A high count can be confounded by error in counting too many small colonies, or difficulty in counting overlapping colonies.Use sterile pipettes for the dilutions, and use different ones in between the different dilutions. To do otherwise will increase the chances of inaccuracy because of carry-over of cells.Accuracy in quantitation is determined by accurate pipette use and adequate agitation of dilution tubes.REFERENCESThere are no references at this time. REVIEWERSThis resource was peer-reviewed at ASM Conference for Undergraduate Educators 2005.
Participating reviewers:Donald Breakwell
Brigham Young University, Provo, UTJoan E. Cunnick
Iowa State University, Ames, IAMichelle Furlong
Clayton State University, Morrow, GAMark Gallo
Niagara University, Niagara University, NYChristopher Woolverton
Kent State University, Kent, OH.